
Technical Tutoring Home · Site Index · Advanced Books · Speed Arithmetic · Math Index · Algebra Index · Calculus Index · Trig Index · Chemistry Index · Gift Shop · Harry Potter DVDs, Videos, Books, Audio CDs and Cassettes · Lord of the Rings DVDs, Videos, Books, Audio CDs and Cassettes · Winnie-the-Pooh DVDs, Videos, Books, Audio CDs, Audio Cassettes and Toys · STAR WARS DVDs and VHS Videos
More Elementary Chemical ReactionsIntroduction · Chemical Bonding and Periodicity · Group 1 (orig. Group IA) Elements – the Alkali metals · Group 2 (orig. Group IIA) – the Alkaline Earth Elements · Group 13 (orig. Group IIIA) Elements · Group 14 (orig. Group IVA) Elements · Group 15 (orig. Group VA) Elements · Group 16 (Group VIA) Elements · Group 17 (Group VIIA) Elements - the Halogens · Afterword · Recommended Books
In the first article on chemical reactions, we looked at the fundamental rule for chemical bonding, namely, that elements bond so that by sharing their electrons, each atom completes its outer electron shell and so looks like a noble gas. The noble gases, which can be found in the far right hand column of the periodic table, have outer shell electron counts of 2, 8, or 18 electrons. For now, we are ignoring the 18-electron case - complete shells have either two or eight electrons in their outer shell.
Chemical Bonding and Periodicity
The examples considered in the previous article included two bonding concepts: no bond (lone pair) and single bonds with two electrons per bond. Lone pairs of electrons arise when the central atom has five or more electrons in the outer shell. The exact configuration of electrons around an atom depends largely on the number of electrons in the outer shell. Columns of the periodic table contain elements with the same number of electrons and are called groups. We will now look at several groups of the periodic table and study their bonding characteristics.
Group 1 (orig. Group IA) Elements – the Alkali metals
Group I elements all have exactly one electron in their outer shell. There is one exception: hydrogen has exactly one electron, period, is not a metal, and is in this group sort of by default. We’ll pretend hydrogen is excluded from the following discussion. The group one elements (except hydrogen) are all soft, highly reactive metals that are never found naturally in pure form. All of them readily give up their single outer shell electron when reacting with another atom that can accept an electron. Thus, by losing an electron, they look like the noble gas that immediately precedes them on the periodic table. For example, lithium loses an electron to look like helium, sodium loses one to look like neon, and potassium loses one to look like krypton, and so on. Losing an electron during a reaction is a bit different than forming a chemical bond. The process is called ionization. The alkali metal becomes a charged ion (in fact it takes a +1 charge) while the other atom involved takes on a negative charge.
Example
Lithium reacts with hydrogen to form lithium hydride. Show the electron transfer that makes this reaction happen.
Solution
There are actually two processes happening simultaneously here. We’ll show them as separate reactions,
but keep in mind that they happen together. "First", lithium loses its outer shell electron:
![]()
then hydrogen picks up the electron (which completes its outer shell):
![]()
Note carefully how the notation works – electrons bound to an atom are dots, but "free" electrons are denoted e-. The lithium has +1 charge and the hydride ion (hydrogen with the extra electron) has –1 charge. They "stick" together because they have opposite charges. This is a case of so-called ionic bonding or bonding due to opposing charges. It is not the same as sharing (although a little sharing goes on, this is not the principal effect) since the hydrogen "monopolizes" the electrons.
We can add the above two equations together and get an overall process. Notice that the free electron appears on both sides of the arrow, and so we cancel it out in the overall equation.
![]()
Group 2 (orig. Group IIA) – the Alkaline Earth Elements
Group two elements have two electrons in their outer shell. Most are soft metals, and some are found pure in nature (particularly magnesium). The are considerably less reactive than the alkali metals, but are still reactive. Considering the ionization of a representative element, calcium, we can see the important difference between these elements and the alkali metals. First, calcium loses one of its two outer shell electrons:
![]()
The second ionization is harder: we’re trying to remove a negatively charged electron from a positively charged ion. Attractive forces between the opposing charges tend to bring them back together. The second ionization looks like this:
![]()
The Ca+2 ion now has noble gas configuration, but work had to be done to get it there. This is a reasonable explanation of why these elements are not as reactive as Group I elements. Another element, such as oxygen, snaps up the two free electrons:
![]()
Adding the above three equations gives us an overall picture of how calcium oxide (lime) is formed:
![]()
Group 13 (orig. Group IIIA) Elements
These elements possess three electrons in their outer shell. The first such element, boron, is rather rare and principally found naturally in borax, used as a detergency-enhancing agent. Boron is used industrially as a semiconductor dopant; certain boron compounds, such as sodium borohydride, NaBH4 and the boranes, BnHm are useful in organic chemistry as reactants. A much more common Group IIIA element is aluminum, which we will discuss later in our section on metals.
Example
For the sake of a simple example here we will study the formation of the borane molecule, BH3, even though this molecule is so unstable that it cannot be isolated.
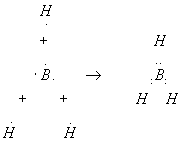
Notice that the boron atom in the center has six electrons – not a complete shell. We cannot really say the hydrogens ionize the boron (take the electrons and make charges), so we are stuck with an incomplete shell. Thus, the instability of this molecule is not too surprising. Stable boron compounds usually have four other atoms bonded to each boron atom:
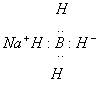 Sodium Borohydride, NaBH4
Sodium Borohydride, NaBH4
or
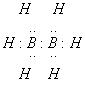 Diborane, B2H6
Diborane, B2H6
In each case, the stable molecules have complete outer electron shells. This happens mostly through sharing of electrons.
Group 14 (orig. Group IVA) Elements
The first two elements of Group IVA each define large industries exclusively dedicated to producing economically beneficial products from the special properties of one atom. Carbon is the basic building block for all organic compounds, including all petroleum products, and all biologically interesting compounds including living systems. Silicon is the basic building block of the semiconductor industry, the rock upon which high technology has been built. For now, we’ll just take a look at carbon and how it typically bonds.
The property that makes carbon different from most other elements is its ability to bond with itself – carbon forms chains which can be thousands of atoms long or more. We shall consider this property in detail when we begin to discuss organic chemistry. For now, we’ll just look at a simple example.
Example
Methane has molecular formula CH4. Show how the bonds form.
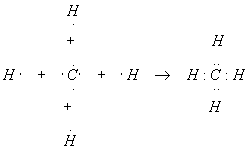
Group 15 (orig. Group VA) Elements
Nitrogen and like atoms in Group VA have five electrons in the outer shell, and so typically form three bonds and one lone pair when combined in molecules. We will have more to say about the way nitrogen bonds when we discuss covalent bonding in detail.
Example
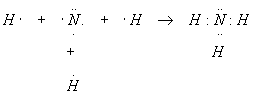
These elements, typified by oxygen, all have six electrons in their outer shell. During reactions, they usually form two bonds and two lone pairs of electrons, as in:
Example
![]()
We will have more to say about the way oxygen bonds when we discuss covalent bonding in detail.
Group 17 (Group VIIA) Elements - the Halogens
These elements, typified by chlorine, all have seven electrons in their outer shell. During reactions, they normally form one bond and three pairs of lone pairs. They are called halogens (salt-forming) elements because they form salts so frequently. These elements really like electrons, and will steal one from another element to complete their outer shell. They often bond in this fashion (ionization) when combining with metals, but when combining with non-metals, particularly carbon and oxygen, they are more likely to share electrons.
Example
![]()
Please see the articles on ionic bonding, covalent bonding, organic chemistry, quantum mechanics and atomic orbitals for more detailed information on the topics covered in this article.

$14.35 · The classic chemistry problem book - very light on theory, plenty of problems with full solutions, more problems with answers

$8.05 · A simplified and updated version of the classic Schaum's Outline. Not as complete as the previous book, but enough for most students